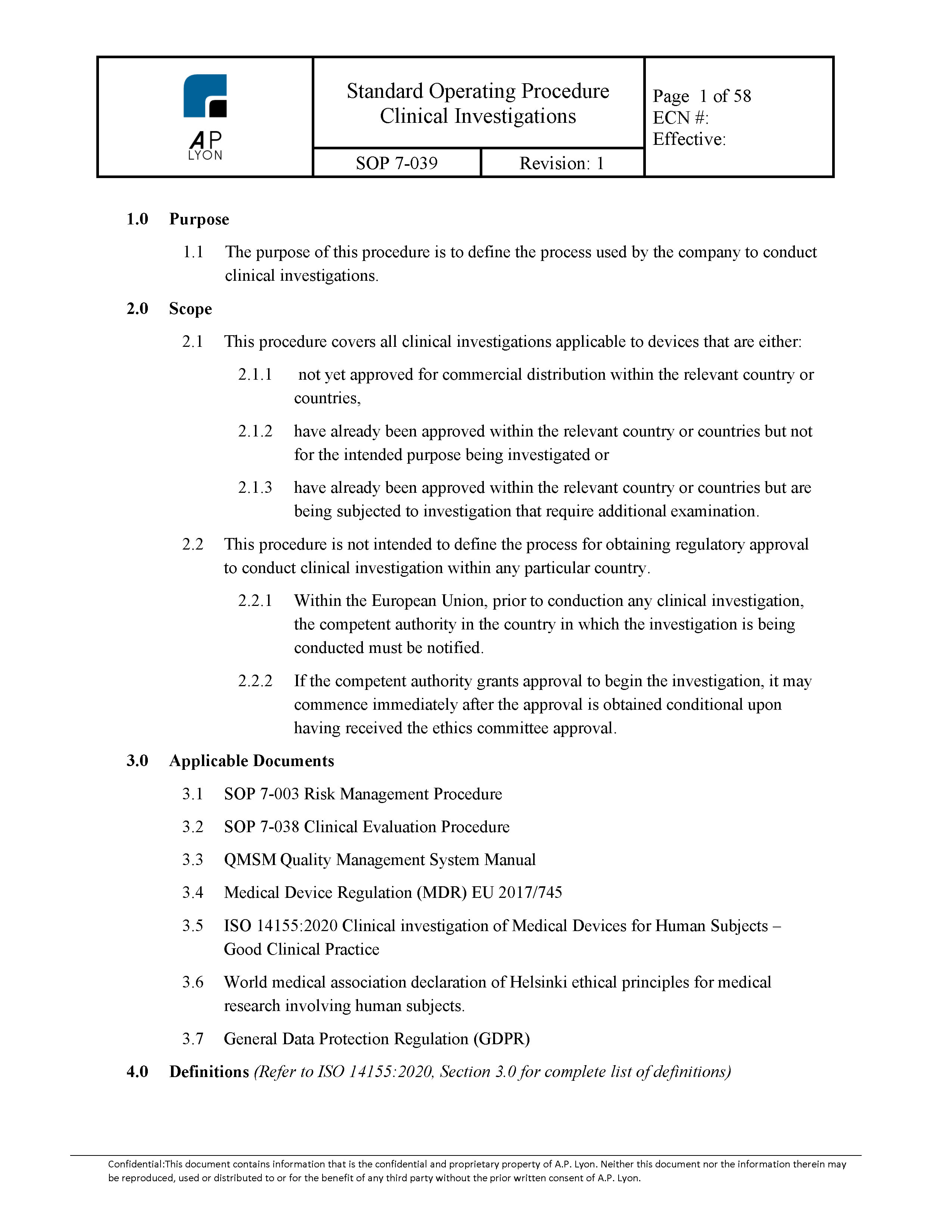
Applications for Medical Device Investigational Testing Authorizations Guidance Document - Canada.ca

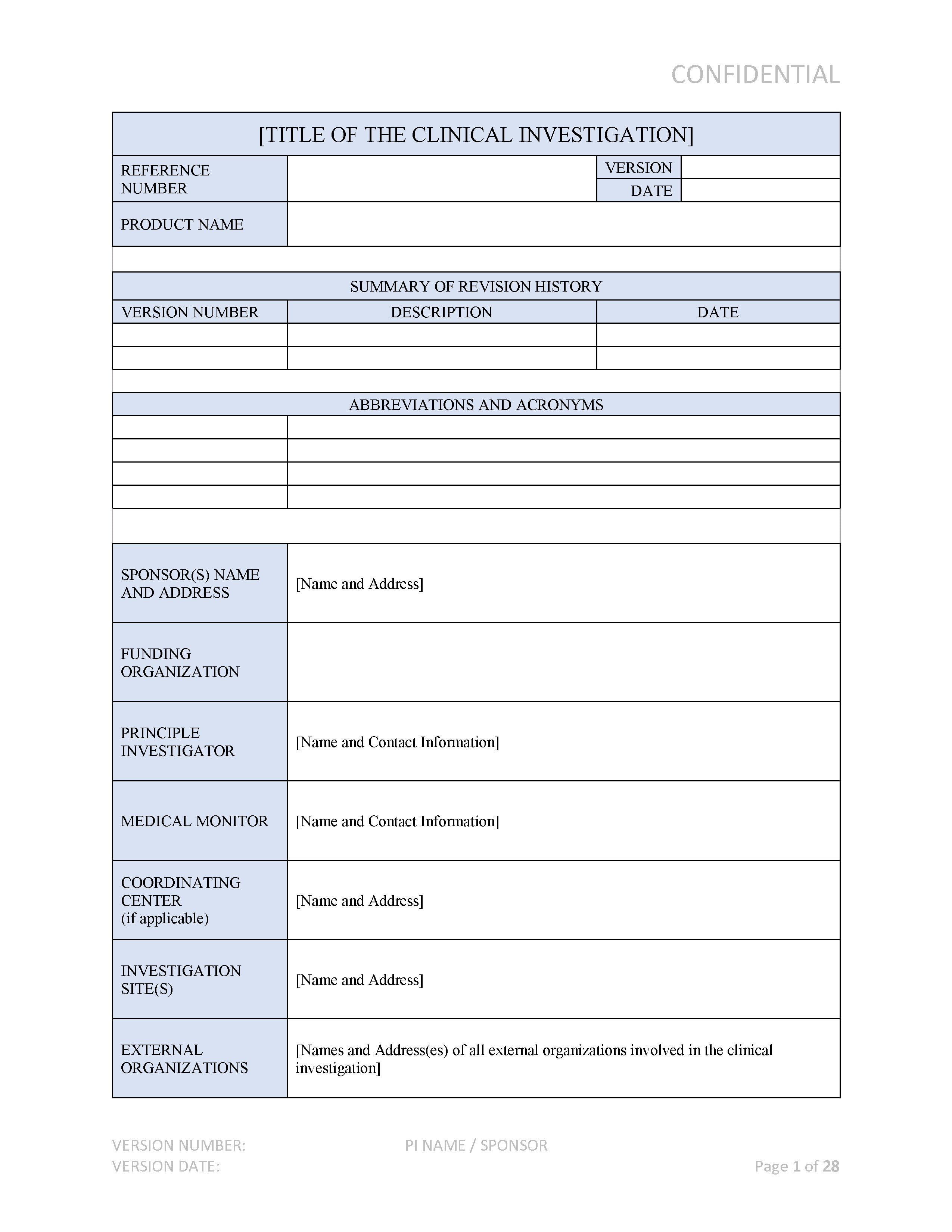
ANSI/AAMI/ISO 14155-2:2003 - Clinical investigation of medical devices for human subjects - Part 2: Clinical investigation plans

ONORM EN ISO 14155-2:2009 - Clinical investigation of medical devices for human subjects - Part 2: Clinical investigation plans (ISO 14155-2:2003)

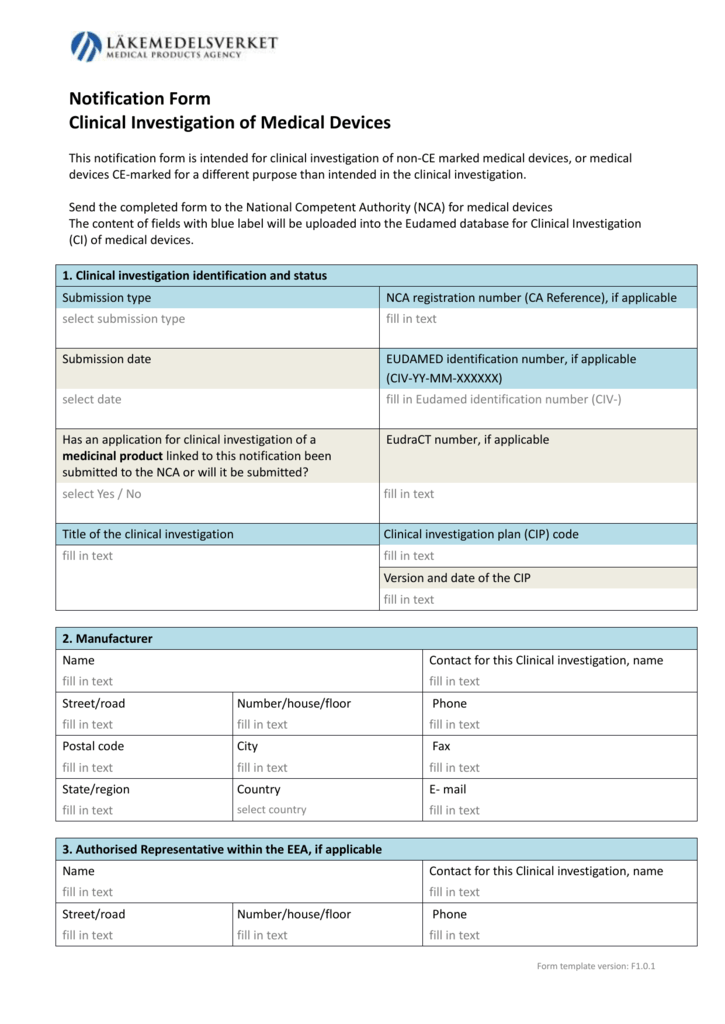
Importance of systematic literature search for clinical evaluation(ce) the strict adherence of medde by PepGra CRO - Issuu