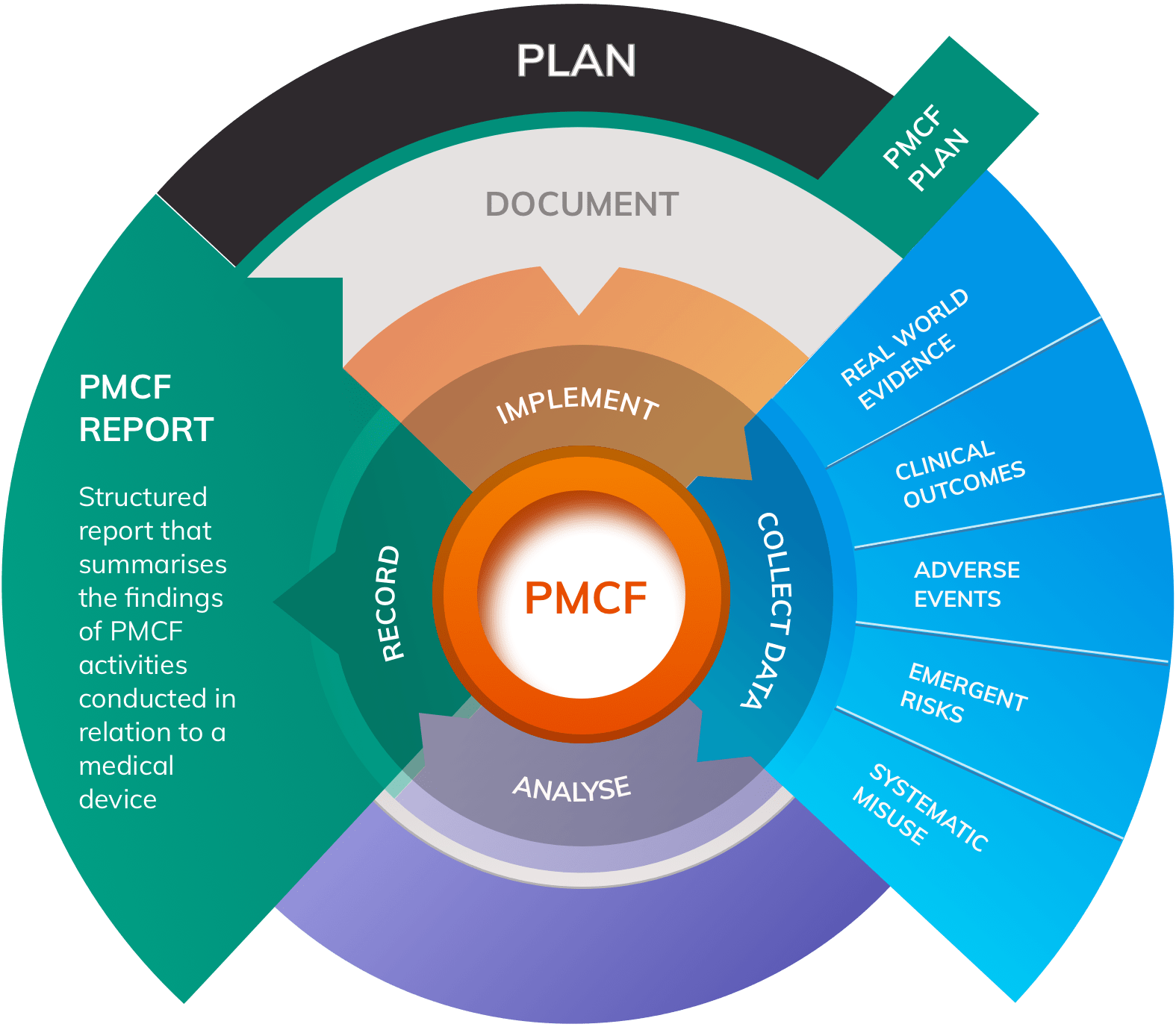
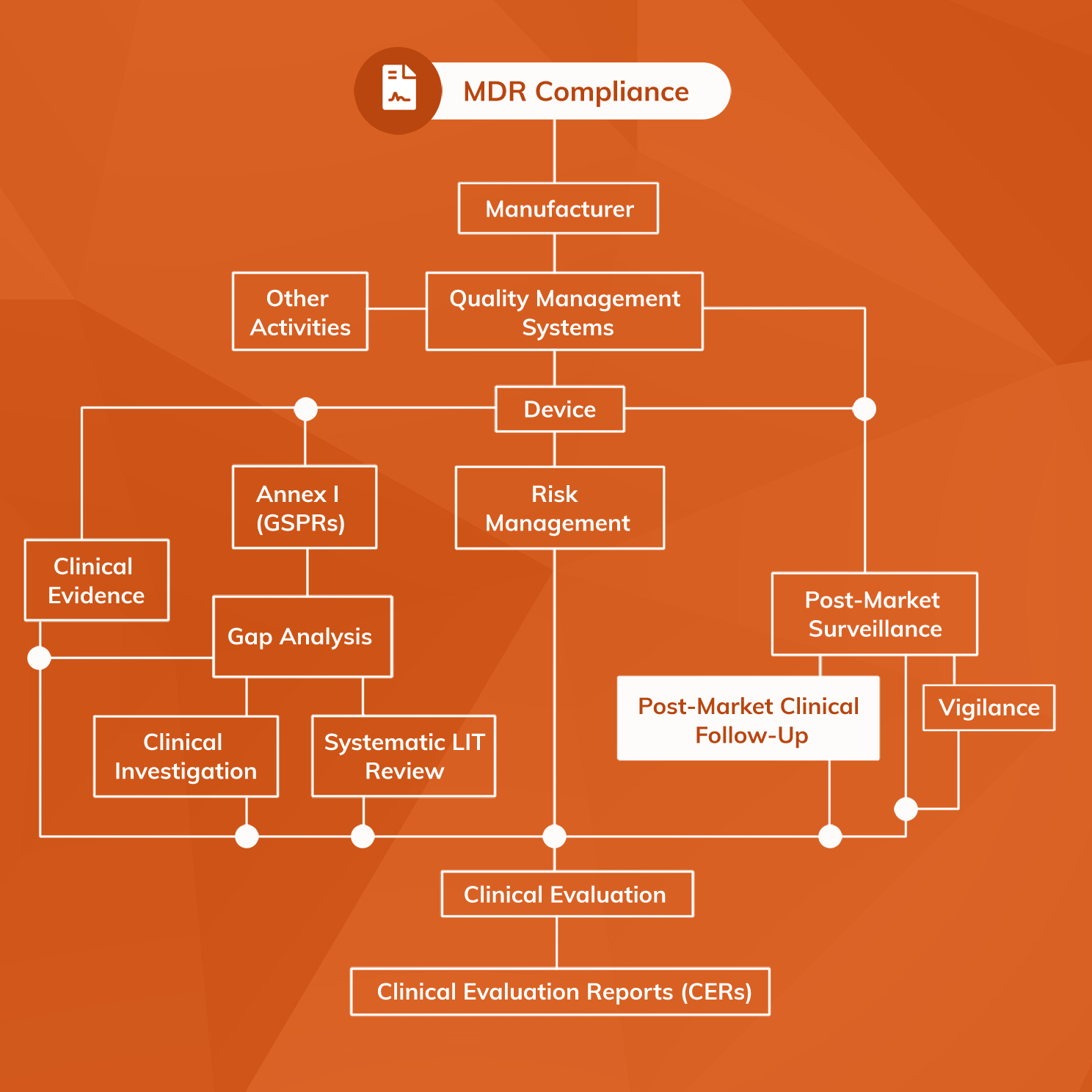
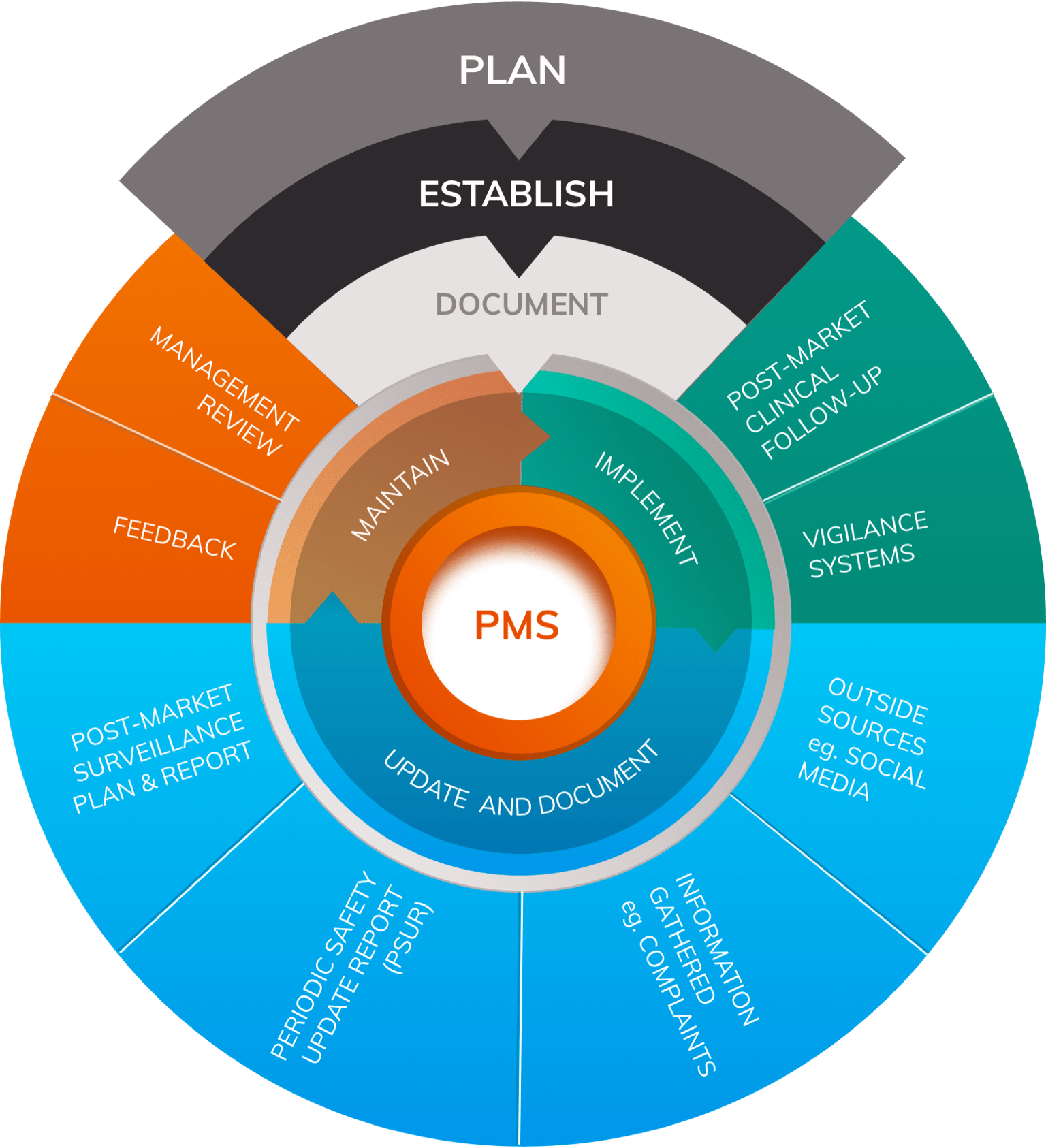
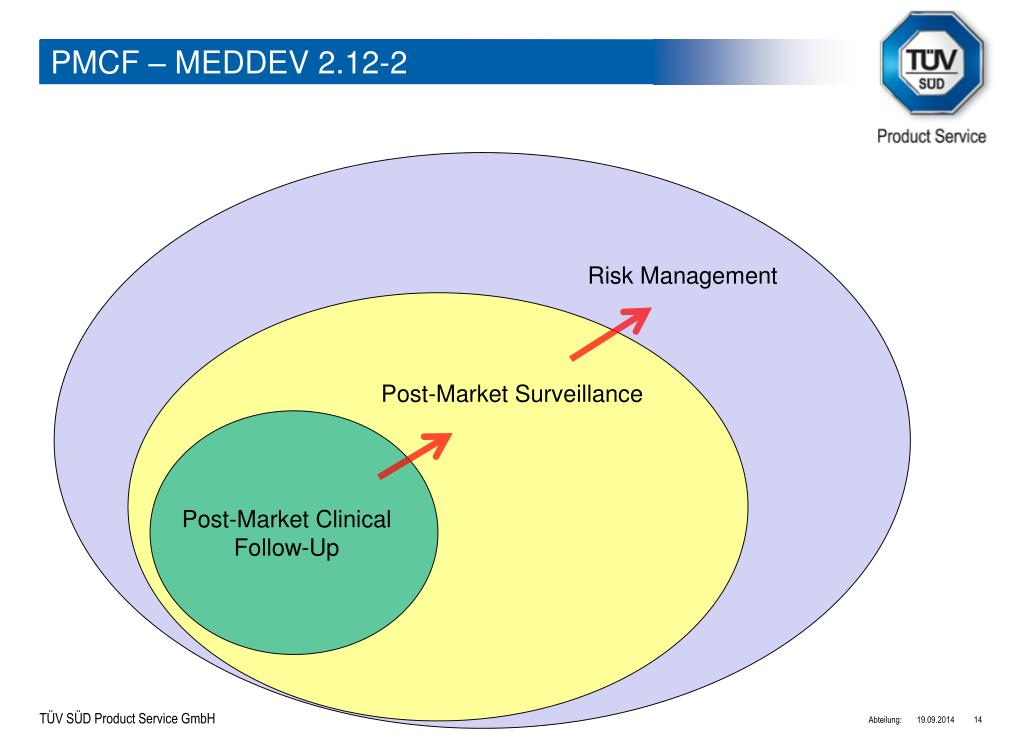
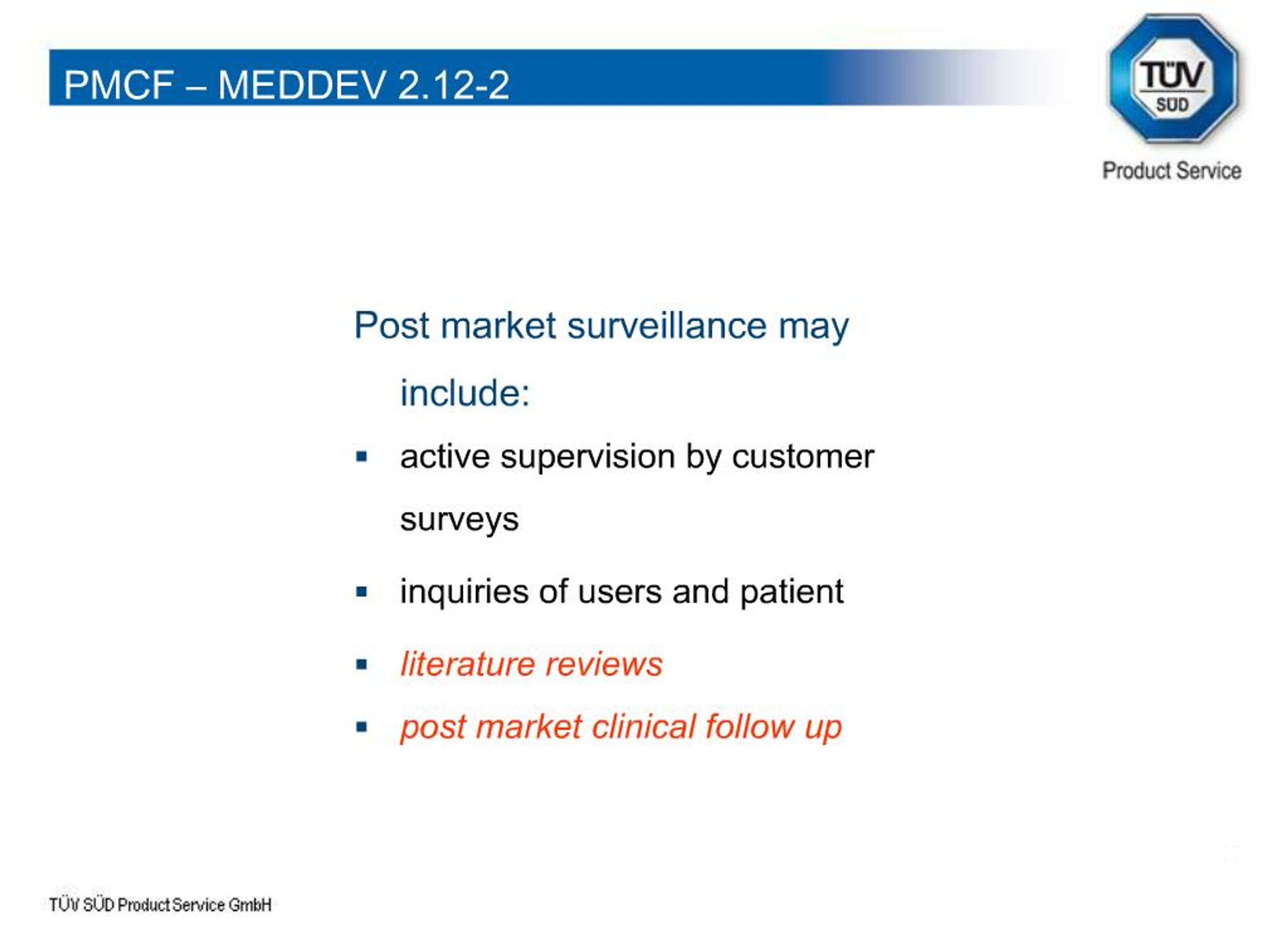
The Post-Market Imperative: Understanding the requirements for effective post-market clinical follow-up - BONEZONE

EU postmarket surveillance plans for medical devices - Pane - 2019 - Pharmacoepidemiology and Drug Safety - Wiley Online Library